May 27, 2020
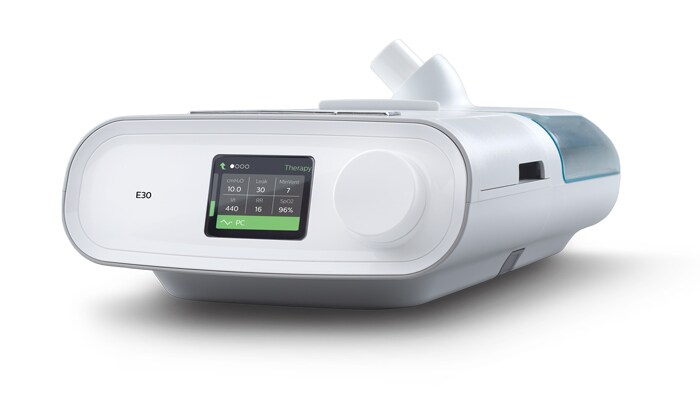
Philips launches Respironics E30 ventilator to aid COVID-19
New ventilator with visual and audible alarms allows for high-flow oxygen to support patients with respiratory insufficiency both invasively and non-invasively
Sydney, Australia – Royal Philips (NYSE: PHG, AEX: PHIA) recently announced its initiative to quickly scale production of its new Philips Respironics E30 ventilator as a readily available ventilation alternative during the COVID-19 crisis in situations where full-featured, critical care ventilators are not available. Production on this device has already commenced, with an initial output of 15,000 units per week in April, ramping up outputs on an ongoing basis. This is now available in Australia, and means that more patients can be treated by making this E30 available with a shorter lead time.
Designed for mass production by a team deeply experienced in respiratory care, the compact and cost effective E30 ventilator features include:
“As COVID-19 continues to spread globally, healthcare providers are working diligently to treat soaring numbers of patients at a time when there are too few ventilators to provide care,” said Matt Moran, Philips Australia and New Zealand Managing Director. “Philips is responding to this pressing global need by quickly scaling production of the new Philips Respironics E30 ventilator with the needs of healthcare workers and COVID-19 patients in mind while also complying with medical device quality standards. Our hope is that this solution will help to free-up ICU ventilators for use in treating the most severe patients.” The Philips Respironics E30 Ventilator is provided globally for use under local emergency use authorizations, such as the FDA Emergency Use Authorization for ventilators, for use in relation to COVID-19, and waiver of CE marking, which authorize its use for the duration of the COVID-19 public health emergency, unless terminated or revoked (after which the products may no longer be used). For more information on the Philips Respironics E30 ventilator, please click here.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 81,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.