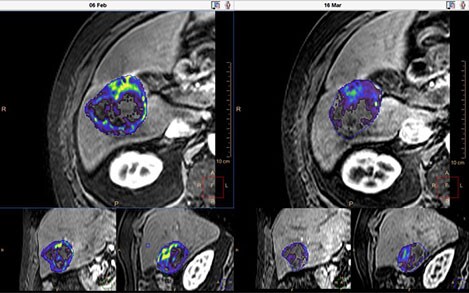
Assessing how a patient is responding to cancer treatment is an everyday clinical task that remains an important clinical dilemma and a key issue in the cancer treatment cycle. The Johns Hopkins Institute research group at the Department of Radiology and Biomedical Imaging at Yale School of Medicine, led by Prof Jeff Geschwind, teamed up with Philips to develop the qEASL* (quantitative method according to the European Association for the Study of the Liver criteria) 3-D tumor quantification. qEASL is a 3D semi-automated method developed for evaluation of treatment response of hepatocellular carcinoma after TACE treatment. It incorporates functional information from contrast-enhanced scans to quantitatively assess the viable tumor volume by giving them a visual indication of how cells respond to therapy.
Toward faster assessment of cancer therapies
The Geschwind research group, part of the leading liver cancer center world-wide, wanted to develop a method to ensure that a patient is on the right treatment plan as early as possible. That means assessing the tumor’s response to the treatment, which in this case was chemoembolization.
All the existing methods for measuring treatment response were based on manual 1D or 2D measurements on axial slices taken from medical images of the tumor. Selection of the slice depends on the reader and it’s difficult to find the same slice levels on follow-up imaging, causing low inter-reader agreement. In addition, tumor heterogeneity and focal enhancement patterns may make these measurements imprecise.
The group started discussing how to make the measurements 3D and more quantitative. This resulted in qEASL, a new semi-automatic, 3D quantitative method that allows researchers to analyze 3D imaging scans (such as CT or MR) with the aim to differentiate between living and dying tumor tissue.
Hitting the right spot in developing clinical tools
A big issue in developing new healthcare tools is that it is not always easy for engineers and technology companies to understand the daily realities you face in working with your patients. If the resulting tools work great in theory but have workflow issues or other mismatches with user needs, people may try them a few times and then stop using them. The tools could then fade from the market even if they have tremendous clinical promise.
The collaboration between health tech companies and the people ‘on the ground’ – clinicians, clinical researchers, clinical administrators etc. – is an approach that should ensure that new tools do meet user needs. Philips has a program where it embeds its own researchers at some of its collaboration partners. The Philips person embedded with Prof Geschwind’s group is Ming De Lin, Senior Clinical Site Researcher at Philips.
Development for clinicians, by clinicians
Julius Chapiro, MD, is co-director of the Interventional Oncology Research Lab at Charité University Hospital, Berlin. He was part of the Geschwind group at the time of the qEASL project, and says: “For me, it was a fantastic experience to be working side-by-side with an Ivy League-based PhD-level engineer. We talked every day, looking at the same clinical issues but from completely different perspectives. All the Philips people we worked with were really keen to listen to and learn from us. They really got to understand the clinical challenges we face in trying to tackle a tumor, and they taught us about the technical options and possible solutions. And throughout the development, we worked as a team. When there were problems we couldn’t solve individually, we could pool our expertise and address them together.”
About the collaboration timeline, he says: “One of the nicest parts about working with Philips was that at no time did they put any pressure on us to speed up development. They let us clinicians take the lead, and develop a strong first evidence-base on the use of the application. We are keen to develop the concept into an official guideline. Here Philips’ large network of clinical partners is a big help and we continue to work as a team along this exciting journey.”
Researchers can now start using qEASL for liver, uterine and brain tumor analysis using a variety of imaging modalities.
*For research use only.