Liver cancer is one of the leading causes of cancer deaths in the world, and one of the most challenging cancer types to treat. Not all liver cancer patients are candidates for surgery, chemotherapy or radiation therapy. Minimally invasive, image-guided interventional oncology procedures may be an option for patients who cannot be treated through other conventional techniques. Around 80,000 interventional oncology procedures were performed in 2013 in the US alone, a figure expected to double by 2023.¹
To help Clinicians to segment tumors and plan and guide interventional procedures, Philips has created the first complete interventional oncology portfolio for interventional radiologists. OncoSuite - All interventional Oncology in one room, is a highly competitive portfoloio with XperCT DUAl, EmboGuide and XperGuide, including Ablation feature.
Innovation and collaboration to get the most out of interventional oncology
Dr. Jeff Geschwind, Professor and Chairman of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, USA and a longtime collaborator of Philips, says “The key is to benefit the patient. This is a message that I have repeated to the hospital administration and that really resonated with them. They understand the impact of having the latest technology available so that the patients benefit.”
The most common interventional oncology procedures of the liver are tumor embolization and ablation. Tumor embolization is a catheter-based procedure where clinicians block the tumor’s blood supply, often combined with localized delivery of chemotherapy (chemoembolization) or radiation (radioembolization). Ablation therapy is performed by percutaneous insertion of needle into the tumor and ablating the tumor, often with radiofrequency, microwave or cryo techniques.
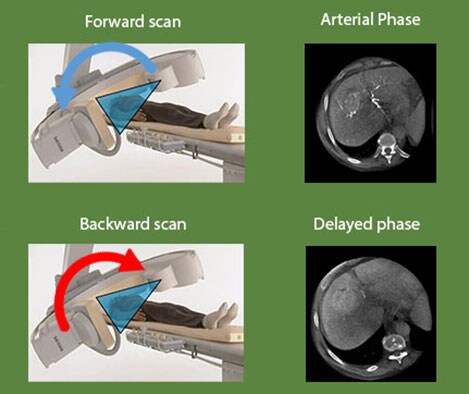
XperCT DUAL cone-beam CT to visualize both the tumor and the feeder vessels
For a successful embolization procedure, it is crucial to have a complete visualization of the size and location of the tumor and all the blood vessels supplying the tumor.
XperCT DUAL provides imaging with almost MRI-like lesion detection. Automatic acquisition of forward and backward scans allows you to visualize the arterial phase, when the contrast agent highlights the vasculature, and the delayed phase, when the contrast agent accumulates in the tumor. Merging these two images in the DualView mode shows both the tumor and its feeder vessels.
Dr. Geschwind says: “The specific role of Dual-Phase cone-beam CT is to improve visualization of tumors, particularly in the liver. Especially for tumors that are less hypervascular, it can be very difficult to identify them and visualize them during the procedure. Obviously, if you can't see the tumor you are about to treat, you may not only miss it altogether but you may also target a large area that could result in destruction of healthy liver tissue.”
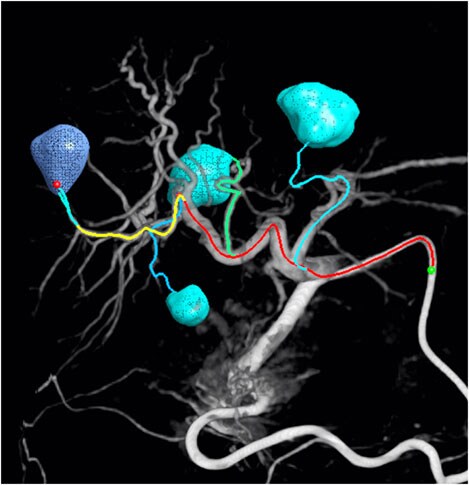
EmboGuide: treatment planning and Live Image Guidance for embolization
The segmented lesions are verified based on the planned position of the catheter tip. During the procedure, Live Image Guidance assists clinicians in reaching each of the verified feeders for a selective or even super-selective embolization.
Dr. Miyayama, Department Head of Diagnostic Radiology, Fukui-ken Saiseikai Hospital, Japan, says: “Trans-arterial chemo-embolization (TACE) is not considered a curative treatment, but with advances and an ultra-selective approach, it can in some cases come close. The physician's burden can be considerably mitigated by use of EmboGuide.” EmboGuide’s feeder detection step helps the clinician to detect even small hepatocellular carcinomas and their feeding branches.
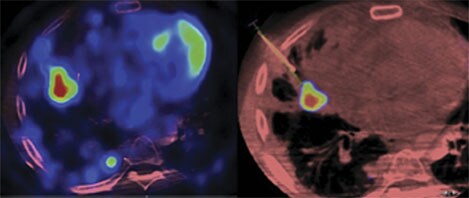
XperGuide Ablation: treatment planning and live needle guidance
What EmboGuide does for embolization, XperGuide Ablation does for percutaneous ablation procedures. It allows reliable and accurate needle interventions for tumor biopsies and ablations, even for locations that are small and hard to reach, at a low dose. In the segmentation step, one or more lesions can be visualized and segmented. Then, the planning step allows you to plan the treatment, showing one or more needle paths for radiofrequency, microwave or cryo-ablation antennas. The tool visualizes specific needle isotherms in relation to the patient’s anatomy to help reduce the risk of compromising adjacent organs. During the procedure, Live Image Guidance feature shows the path of the needles and real-time antenna position, allowing clinicians to course-correct and guide the needle to the target position.
Interventional oncology in the future
With the tools and features available in OncoSuite, treatment planning for interventional oncology has already become visual and accurate. Clinicians can now visualize the projected result of a treatment and manage the risk of damaging healthy tissue. In the area of imaging, 2016 will see the release of OncoSuite 2.0, with even more features to support clinicians in planning and performing interventional oncology procedures.
Radio-opaque embolization beads are another exciting development in the field of interventional oncology. Philips’ collaboration with BTG, a global specialist healthcare company, and their recent announcement on the first patient treated with radiopaque beads is a significant milestone and stands as testimony to Philips’ contribution in the field of interventional oncology.
Dr. Geschwind clearly points out: “I think the three areas of imaging, drug delivery and the drugs themselves are really the future. I think in the next 5 to 10 years, we’re going to see an explosion in these fields of research that may coalesce to give us a significant advance in patient care.”
¹ Based on data from Medtech 360 Report: Interventional Oncology Devices | US | 2015 | Market Analysis, 2015 Decision Resources Group.
For more information
Read more about the Philips Interventional Oncology solutions here:
Contact: Claus Schaffrath, MD MSc Segment Lead - Interventional Oncology IGT Systems Marketing Product Manager - Interventional Oncology IGT Systems Marketing
Thiru Kanagasabapathi, PhD
Written by:

Thiru Kanagasabapathi, PhD Product Manager - Interventional Oncology IGT Systems Marketing