With an aging population, large joint replacements and cardiac implantable devices are becoming increasingly prevalent. Also, the prevalence of conditions needing an MRI examination, such as neurodegenerative diseases, cancer and musculoskeletal diseases, increases with age. Not all implanted devices are an absolute contraindication for MRI anymore. Patients with MR Conditional implants can undergo MRI, but only under clearly defined conditions and performed by well-trained staff. In this article, four experts discuss scanning patients with MR Conditional implants.

In five major European countries (France, Germany, Italy, Spain and the UK) more than 2.5 million large joint reconstructions and spinal implant procedures were performed in 2015, a figure expected to rise to more than 3 million in 20201.
In those same countries, the prevalence of such passive orthopedic implants was 19% among people over the age of 65 years, which is predicted to rise to 30.5% in 20201. The prevalence of active cardiac implants is also expected to rise from the current 10.4% in the over 65 population to 11.8% in 2020.
2015 | 2020 | |
# Procedures | > 3 M | > 3.4 M |
Total population | 321 M | 326 M |
% with implant | 3.9% | 7.2% |
Total 65+ | 63 M | 68 M |
% 65+ with implant | 13.3% | 28% |

2015 | 2020 | |
# Procedures | > 2.6 M | > 3.1 M |
Total population | 322 M | 345 M |
% with implant | 3.1% | 5.6% |
Total 65+ | 48 M | 56 M |
% 65+ with implant | 21.6% | 31.3% |

A similar situation is seen in the USA where 17% of the over 65 population are estimated to carry a large joint or spinal implant and 10.4% have a cardiac implant, figures predicted to rise to 26% and 11.8%, respectively, by 20201.
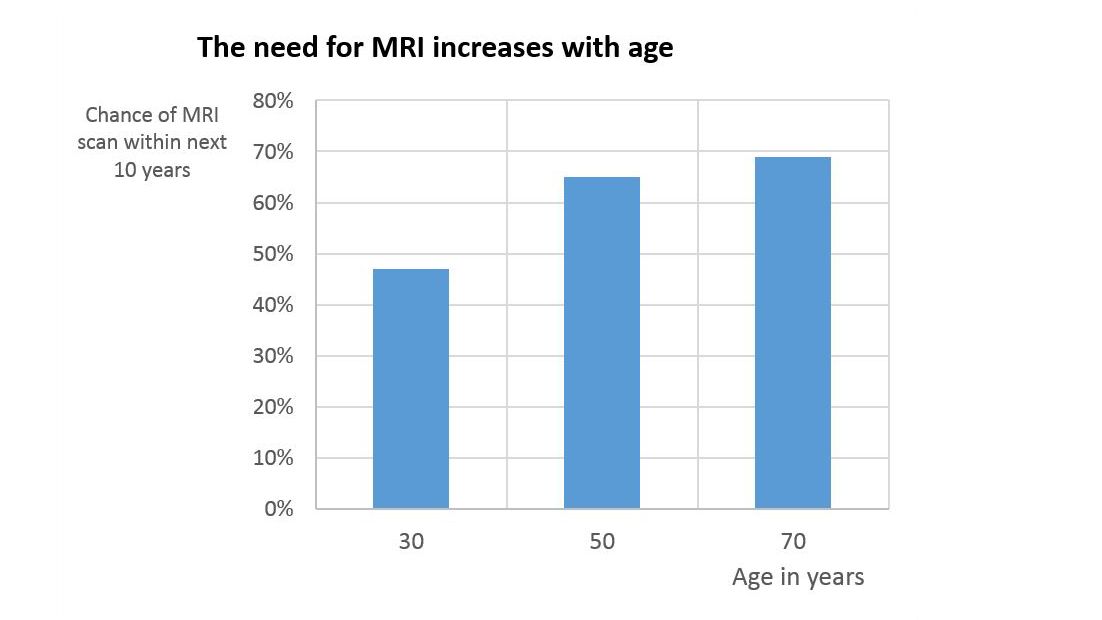
There is a significant clinical need for patients with orthopedic implants to undergo MRI examinations. Besides the increased prevalence of medical implants in older people, the likelihood that the average person will need an MRI scan during the next 10 years increases with age from around 47% at the age of 30, to around 69% at the age of 701.
Combining these numbers with the data on implant incidence suggests that, for instance, in the USA, 5.7 million patients over 65 and carrying orthopedic or cardiac implants will need an MRI scan within ten years. And this will rapidly rise to about 12.6 million patients in 2020, a doubling of the number in five years.
A recent USA-based study of patients with spinal cord stimulation (SCS) implants estimated that about 82–84% of SCS-implanted patients will need a spinal MRI scan within five years of receiving their SCS implant. A further 59–74% of patients will need a non-spinal MRI scan within ten years2.
There are only few implants for which scanning is an absolute contraindication.

There are currently over 34 million MRI patient exams per year within the USA [3]. About 3.9% of the US population – 12.5 million people of whom 10.3 million are over 65 – are presently carrying an orthopedic or cardiac implant. Due to safety concerns, patients carrying some kind of metallic medical implant or device may potentially be denied an MRI scan. But are such concerns justified?
“There are only few implants for which scanning is an absolute contraindication, but we can scan patients with an MR Safe or MR Conditional implant” says Emanuel Kanal, MD, Director of Magnetic Resonance Services and Professor of Radiology and Neuroradiology at the University of Pittsburgh Medical Center, USA. As Dr. Kanal explains, many patients who are referred for an MRI scan have an implant of some kind. “At an academic center the chances of a patient having an implant are much higher than at free standing, private practice environment. I would guestimate that at our site the number of patients with implants is somewhere between 10% and 25%.”
According to Greg Brown, MRI technologist studying at the Centre for Advanced Imaging, University of Queensland, Australia, a similar situation exists in his country.
“There may be certain devices or implants that at certain levels of radiofrequency power may be potentially dangerous to scan,” says Dr. Kanal. Such implants may interfere with the MRI-related RF fields inside the body resulting in increased risks to the patient due to local hot spots.
“An MR safe implant has no potential interaction with a scanner,” says Mr. Brown. “So that would be non-conducting, non-magnetic objects. But other implants have the label ‘MR Conditional’ and that term is really quite important.”
All medical implants have to be tested by their manufacturers for MR safety and labeled according to standardized terminology4.
Active implants are those that contain a power source (such as cardiac pacemakers and spinal cord stimulators), whereas passive implants have no power source (such as aneurysm clips and replacement joints).

*Reprinted, with permission, from ASTM F2503-13 Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment, copyright ASTM International, 100 Barr Harbor Drive, West Conshohocken, PA 19428.
MR Safe: An item that poses no known hazards resulting from exposure to any MR environment. MR Safe items are composed of materials that are electrically nonconductive, nonmetallic, and nonmagnetic.
MR Conditional: An item with demonstrated safety in the MR environment within defined conditions. At a minimum, address the conditions of the static magnetic field, the switched gradient magnetic field and the radiofrequency fields. Additional conditions, including specific configurations of the item, may be required.
MR Unsafe: An item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment.
Although comprehensive guidelines for the safe use of MRI have been issued by professional bodies such as the American College of Radiology (ACR)5 and Medicines and Healthcare Products Regulatory Agency6, some confusion remains in everyday practice. “As a result, patients with implants who need an MRI scan may be either never referred or are denied the scan, this is very much dependent on the site,” Mr. Brown explains.
“Patients with implants are denied MRI scans by some places. But as a major hospital site, we might be looking at just a couple of patients that we really can’t scan, maybe 1-2%. At a site where they are not as comfortable with the safety aspects or don’t want to spend the time on it, they might be rejecting more.”
“The lack of awareness by referring physicians and even radiology experts can be problematic,” says Dr. Kanal. “Many times people tell us they didn’t even bother sending their patients with conditional implants for an MRI. Instead, they sent them straight for a CT, thinking they could not get safely scanned on MRI.”
Blanket rules, like not scanning pacemakers, are not serving the patients very well.
“I think there is a lack of understanding among radiologists and technologists, let alone referring physicians,” says Dr. Kanal. “But I don’t believe it is the referring physicians’ responsibility. I think it is the responsibility of radiology to educate them and to explain that we can perform an MRI on a certain patient by scanning under certain specific conditions.”
“The perception that all active implants, such as pacemakers cannot be scanned, is also incorrect,” says Mr. Brown. “This may still refer back to old guidance coming from early practice. The British Heart Rhythm Society has just released guidelines for scanning MR Conditional pacemakers7. And there has been a 2015 German publication on the same topic8. Both are trying to say that we need to think more about this, because the patients are going to need these scans. So blanket rules, like not scanning pacemakers, are not serving the patients very well.”
Scanning patients with MR Conditional implants inevitably brings its own challenges.
“Scanning patients with MR Conditional implants inevitably brings its own challenges,” explains Paul W. de Bruin, PhD, medical physicist in the Radiology Department at Leiden University Medical Center, the Netherlands. “It involves two steps that cost us time. The first one is figuring out exactly which implant the patient has. That could be improved by having some sort of registry to look up which implant is in the patient. The second is that it can sometimes be difficult to find the information from the implant manufacturer. For instance, when we find that a certain implant is labeled MR Conditional, it means that we can scan under certain conditions. We then have to figure out what those conditions are by going to the implant manufacturer’s website. But it’s often not straightforward to find the information. After that we need to pay special attention when we bring the patient to the scanner and when setting the sequence parameters at the scanner to remain within the condition limits throughout the exam.”
These suggestions are reinforced by Mr. Brown. “The means to rapidly identify the exact device in a patient would help MR sites. A central internet-accessible repository by manufacturers of the instructions for use and MR conditions available for all their devices, including ones no longer sold, would speed things up a lot for sites.”
“Scanning patients with implants means having to be aware of the possible hazards,” says Harald Kugel, PhD, MR physicist at the Institute of Clinical Radiology, University of Münster, Münster, Germany. “There are specific limits on specific parameters. One of the most common limits is on specific absorption rate (SAR)9. In general, this means that spin echo and TSE sequences should be avoided by switching to gradient echo instead. This is just an example of what needs to be done or considered.”
“We have specific standard operating procedures, telling our staff what to do with specific implants. For common implants everybody in our team knows what to do. For instance, if a patient with a pacemaker comes for an MRI exam, we have a specific routine to check which type of pacemaker it is, to check that a cardiologist is present as an MR Conditional pacemaker usually needs to be switched in an MR-compatible mode, etc. So some actions have to be taken and this is all laid down in our procedure.”
Scanning patients with implants means having to be aware of the possible hazards.
“I think a lot more education of technologists and radiologists is needed, so that we can develop an efficient and structured way forward,” Mr. Brown says.
“We know that MRI scanners have to pass certain levels of safety and show that they are documented to be kept at certain guidelines and thresholds10. MR site accreditation in the USA11 documents that the site is appropriately designed.” says Dr. Kanal. “But who is missing in all this? There was no certification process to show that the magnetic resonance medical director, radiologist, technologist or physicist have a comprehensive understanding of the safety issues associated with magnetic resonance environments or how to apply them. We therefore created the American Board of MR Safety in 2014. Its sole purpose is to certify and credential MR medical directors and MR safety officers who represent the radiologists/physicians, technologists, and physicists who are charged with overseeing safety in clinical and research magnetic resonance environments.”
“Sometimes patients have certain implants and the site is not sufficiently familiar with what can and can’t be done to decrease the risk of an MRI scan,” says Dr. Kanal. “They may choose, for ostensible ‘safety’ objectives, to not scan that patient. I put the word safety in quotes because not scanning a patient for whom a diagnostic MRI was requested has its own risks. The patient may go undiagnosed or may have to be sent for a more invasive study to make a diagnosis.”
“Saying ‘no’ is easy, but saying ‘yes’ requires knowledge, confidence in that knowledge, and the willingness to say yes and to apply that knowledge.”
Not scanning a patient for whom a diagnostic MRI was requested can also potentially impact patient care.
He is Chairman of the American Board of Magnetic Resonance Safety and is lead author of the American College of Radiology’s White Paper on MR Safety and MR Safe Practice Guidelines.
He is an Honorary member of the SMRT, and serves on their MR safety group. He is a non-voting Board Member and SMRT delegate on the American Board of Magnetic Resonance Safety, and holds their MRSO certification. Mr. Brown is an active user of social media platforms on the use of MRI and MRI safety.
As a member of the Clinical Physics Group and the C.J. Gorter Center for High-field MRI at Leiden University he has various research interests in the field of MRI including high-field MR (3T/7T) applications, DCE-MRI, sodium MRI, pharmacokinetics, and musculoskeletal applications.
He is the author of numerous articles on MR applications and safety, has been actively involved in the teaching of practical ISMRM courses in MR safety, and is a member of the Technical Committee 'MR Procedures' of the German Standards Committee Radiology.
Scanning patients with Conditional implants
MR ScanWise Implant for MR Conditional implants
Explore how you can confidently expand MR imaging services to a subset of the patient population – patients with MR conditional implants.

